01 Feb 2023
HKU biologists designed and developed novel anti-allergic drugs by targeting Mas-related G protein-coupled receptor (GPCR) X2 (MRGPRX2)
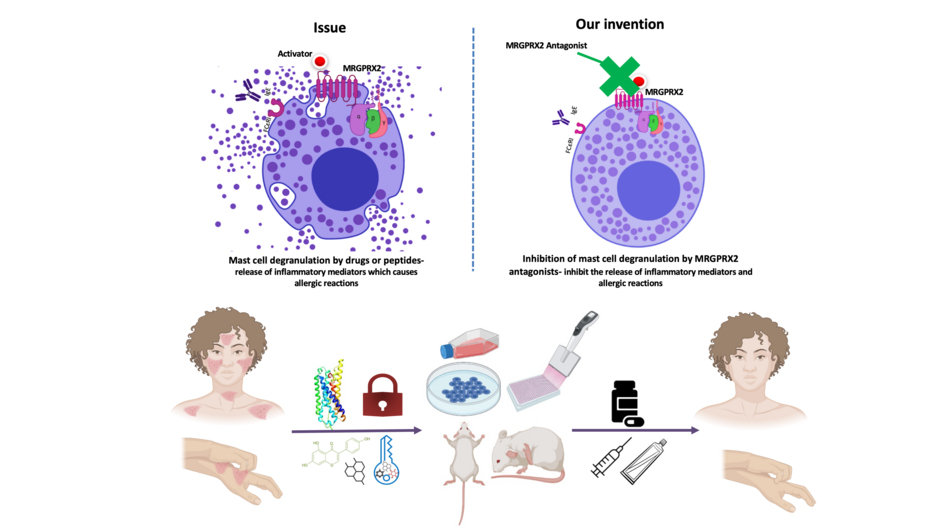
Inhibition of mast cell degranulation by MRGPRX2
Allergic diseases are increasing at an alarming rate across the world. Despite available treatments, several cases of allergies are untreated and cause a huge disease burden. Moreover, allergies cause discomfort and life-threatening threats and can deprive one of taking life-saving vaccines.
A research team led by Professor Billy K C CHOW from the School of Biological Sciences, Faculty of Science, the University of Hong Kong (HKU), have designed and developed novel small molecule MRGPRX2 antagonists which can be developed as novel anti-allergic drugs. This study has recently been published in The Journal of Allergy and Clinical Immunology (JACI).
Research background
In the area of allergy, previously, research on both mast cell degranulation and host response mechanism was focused on the IgE receptors (FcεRI)-mediated immune response. However, investigating the alternative non-IgE mechanisms of mast cell degranulation and allergic reactions has gained immense interest recently. Accumulating evidence on MRGPRX2 has shown its critical role in drug-induced allergic reactions, hypersensitivity reactions, and inflammatory diseases. Mast cells are tissue-resident innate immune cells, present predominately at exterior environment barriers, such as the skin and the respiratory tract. Mast cells are known for their densely packed secretory granules. On activation via activators (allergens, infectious agents, toxins, and drugs), mast cells can rapidly release these inflammatory mediators- this process is known as mas cell degranulation. This lead to allergies and inflammation of surrounding tissues or the whole body (See the diagram).
We have recently published a comprehensive review on the emerging role of Mas-related G protein-coupled receptor (GPCR) X2 (MRGPRX2) as a novel GPCR responsible for non-IgE mediated allergies and other inflammatory reactions. Several FDA-approved drugs, such as fluoroquinolone antibiotics, neuromuscular blockers, Icatibant, opioids, and diagnostic agents, have been reported to cause allergic and inflammatory reactions via MRGPRX2 activation.
Also, recently we have reported the mechanism of allergic reactions induced by a peptide P17 isolated from ant venom and the anti-allergic activity of the flavonoid compound genistein. In the current research, based on the lead structure (genistein), we have designed potent novel small molecule MRGPRX2 antagonists. These novel small molecule antagonists bind with MRGPRX2 (like a lock and key) and inhibit mast cell degranulation and release of inflammatory mediators; thus can be developed as novel ant-allergic drugs.
Key findings
We used a combined approach of structure-aided drug designing, virtual screening, medicinal chemistry, and experimental pharmacology, often used in big pharma for drug development. After screening thousands of molecules, we shortlisted six potent novel small molecules, designed the synthetic schemes and successfully synthesized and characterized them. These molecules are novel in structure and, to our best knowledge, have never been reported. Furthermore, we have screened these novel molecules via our well-established human mast cell model and mice models of acute and systemic allergies. Also, we have tested the anti-allergic potential of small molecules against FDA-approved drugs such as Fluoroquinolone antibiotic ciprofloxacin. The novel small molecule has shown a potent anti-allergic activity.
The societal impact of the findings
The current state of research implies the crucial role of MRGPRX2 in non-IgE mediated allergic reactions, drug-induced allergies, pruritis, and inflammatory skin diseases. The current treatment strategies encompass mainly the symptomatic use of medications like anti-histaminic, corticosteroids, and monoclonal antibodies against IgE receptors. However, several cases of allergies are refractive to currently available treatments and fail to treat acute to severe cases. Moreover, no MRGPRX2-specific and effective drug is available in the market, indicating an unmet demand for drug development.
The research outcome from our project holds promising potential in the domain of mast cell biology and drug discovery and development as it has a direct pharmacological implication. The novel small molecule MRGPRX2 antagonist can be used as the experimental antagonist to understand the mast cells -MRGPRX2 mediated pathways and pathophysiology and can further be developed as a therapeutic drug molecule for the treatment of non-IgE mediated allergic reactions, itch, and inflammatory disorders.
Based on the novelty and commercial impact of these small molecules, we have filed a US patent and a Chinese patent with the help of the technology transfer office of the University of Hong Kong. It is anticipated that our findings possess tremendous therapeutic and social impact.
"Our translational research has opened a novel therapeutic area for treating chronic allergies," said Professor Chow. "I hope our efforts and others in this field will bring therapeutic solutions for chronic allergies in the coming years," said Dr Kumar.
The research was conducted by the team led by Professor Billy K. C. CHOW(On the right). Postdoctoral fellow Dr Mukesh Kumar (on the left) from Professor Chow's group is the paper's first author.
Research Team
The research was conducted by the team led by Professor Billy K. C. CHOW. Dr Mukesh Kumar (Postdoctoral fellow) from Professor Chow's group is the paper's first author. Dr Karthi Duraisamy from Prof Chow's group, Dr Rajasekar Reddy Annapureddy from Ludwig-Maximilians-Universität München, Germany and Dr Chi Bun Chan from the School of Biological Sciences, HKU are the other collaborator and contributed to the research. This research has been supported by the Hong Kong government RGC GRF 1711320, NSFC/RGC 17111421 and HKU seed fund for basic research 201910159222 and 104006152 to Prof Billy K. C. Chow.
About Professor Chow
Professor Billy K. C. Chow is a Chair of Endocrinology at the School of Biological Sciences, HKU. His expertise lies in GPCR and ligand interactions, eventually leading to deciphering the physiologic actions of GPCRs and identifying novel GPCR agonists/antagonists as therapeutic solutions. He is the founder of an HKU spin-off company PhrmaSec Ltd, which aims to translate basic research into societal impact by targeting GPCRs.
The Journal can be accessed here.
Click here for further reading.