13 May 2025
HKU Biologists Identify the protein DNM1 as a Key Regulator in Ovarian Cancer Metastasis
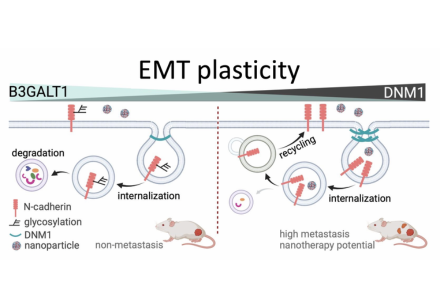
Diagram illustrating how DNM1 regulates EMT and metastasis. Image adapted from BioArt.
Ovarian cancer remains the leading cause of death among cancers affecting the female reproductive system, largely because current treatments are not effective once the cancer has spread (metastasised) beyond the ovaries. A recent study led by Professor Alice WONG, Interim Director from the HKU School of Biological Sciences of The University of Hong Kong (HKU), has identified a critical factor driving this metastasis, offering promising new directions for targeted treatment strategies. The team’s findings have just been published in Protein and Cell.
The researchers focused on a biological process called epithelial-to-mesenchymal transition(EMT). This process makes cancer cells more flexible and able to move, which helps them spread and makes the disease harder to treat. Scientists have struggled to find a part of this process that can be targeted with drugs. To tackle this challenge, the team employed a master regulator (MR) algorithm to analyse regulatory networks of gene and protein interactions. By analysing data from over 8,000 patient samples across 20 cancer types in The Cancer Genome Atlas (TCGA), they identified dynamin 1 (DNM1) as a novel, non-transcriptional regulator of EMT. Unlike traditional regulators that control gene expression and are difficult to target, DNM1 acts through alternative cellular mechanisms, presenting novel treatment opportunities. Notably, higher levels of DNM1 were found in patients with advanced disease and mesenchymal subtypes, and these elevated levels were associated with worse survival outcomes. This discovery suggests that targeting DNM1 could be a valuable strategy to improve outcomes for these patients.
Experimental Insights
In cell-based experiments, inhibiting DNM1 in aggressive ovarian cancer cells significantly reduced their ability to migrate. It also lowered levels of N-cadherin, a key EMT marker linked to aggressive cancer behaviour. On the other hand, increasing DNM1 in non-metastatic cells made them more invasive and increased N-cadherin levels. Animal studies also confirmed that reducing DNM1 led to less cancer spread within the abdomen, reinforcing DNM1’s role in promoting metastasis. The researchers discovered that DNM1 works by helping cancer cells take in (endocytose) and reuse (recycle) N-cadherin, a process that maintains cell polarity and enables migration. These functions are crucial for allowing cancer cells to become more mobile and invasive, contributing to their spread throughout the body.
Notably, the integration of ATAC-seq and RNA-seq analyses showed that non-metastatic cells exhibited higher expression of the glycosyltransferase gene B3GALT1, potentially inhibiting EMT by reducing N-cadherin recycling. Intriguingly, metastatic cells with high DNM1 were more efficient at taking up nanoparticle-based drugs, suggesting a potential advantage for targeted nanomedicine.
Conclusion and Future Directions
Overall, these findings establish the DNM1-N-cadherin axis as a critical regulator of EMT-associated ovarian cancer metastasis and suggest its potential as a biomarker for targeted nanodrug therapy. This research not only enhances our understanding of the mechanisms driving ovarian cancer but also opens new avenues for developing effective therapeutic strategies against this aggressive disease.
For those eager to explore this fascinating research further, the full details can be found in the journal Protein and Cell under the title ‘Dynamin 1-mediated endocytic recycling of glycosylated N-cadherin sustains the plastic mesenchymal state to promote ovarian cancer metastasis’ .
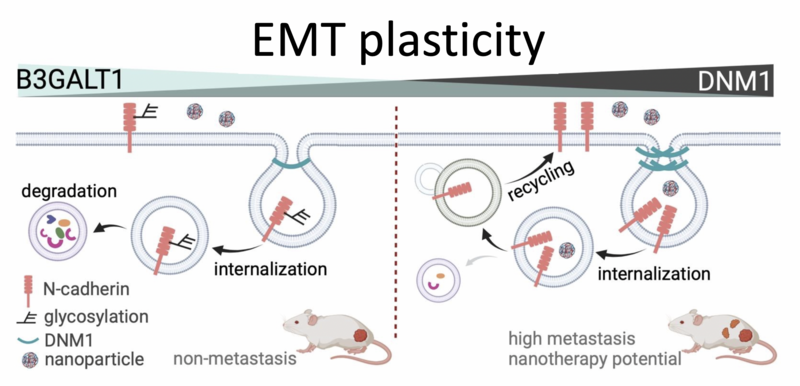
Diagram illustrating how DNM1 regulates EMT and metastasis. High levels of DNM1 enhance N-cadherin endocytic recycling, leading to increased metastasis and greater potential for nanotherapy. In contrast, elevated B3GALT1 levels reduce N-cadherin expression in non-metastatic tumors, which limits their metastatic ability. Image adapted from BioArt.