12 Jun 2024
Sharpening a Solarceramic Stone to turn water into fuel
The Sorcerer’s Stone in Harry Potter can “create the elixir of life or turn metal into gold”. Similarly, a special “Solarceramic Stone”, scientifically known as a Bi4Ti3O12 (BTO) nanosheet, can turn “the elixir of life”, aka water, into hydrogen fuel using sun light. This process becomes over two hundred times efficient if the material is “sharpened” or harnessed into an “H-shaped” structure - with the middle layer partially etched away.
This isn’t magic or “Harry Potter”! In fact, it is a solar-energy-driven “photocatalytic” process, whereby solar light shines onto a semiconductive material to energise its low-lying electrons (in the valence band) to a high-energy level (into the conduction band). Such high-energy electrons can then be used to catalyse other substances, such as water to produce hydrogen. However, the efficiency of the process is practically constrained because those solar-generated electrons are too slow to migrate to the active catalytic site, but too quick to bounce back to their initial positions - without doing anything useful. The challenge is to rapidly draw the electrons to the active sites to catalyse useful reactions.
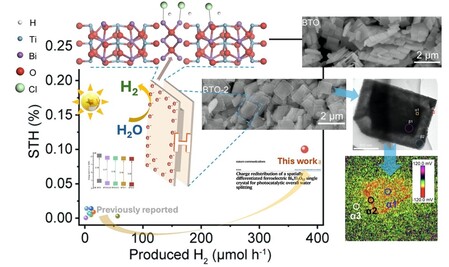
In a recent publication in Nature Communications, Professor Zhengxiao GUO’s group and collaborators have discovered that a BTO nanosheet can be preferentially etched to form a spatially differentiated “H” shaped structure, with the middle-layer etched partially to create a homojunction between the ultrathin edge region and the unetched central core.
This homojunction helps facilitate the transfer of electrons between the edge region and the core region more efficiently. In so doing, the researchers discovered that this “all-edge sharpened” structure overcomes the limitation of electron transfer across different crystal boundaries, so that the solar-excited electrons can be preferentially transferred and accumulated over the surface of the edge region to enhance the overall water splitting for hydrogen generation. Complementary experimental characterisations and electronic structural simulations have clearly identified the redistribution of charges to confirm the above effect.The resulting spatially-differentiated “H-shaped” photocatalyst works effectively for overall water splitting without the use of any sacrificial agent. It produces 40.3 μmol h-1 of hydrogen and 20.1 μmol h-1 of oxygen at a near stoichiometric ratio of 2:1, with a solar-to-hydrogen efficiency of 0.1%, which is over 210 times greater than the pristine BTO under simulated solar light. Further engineering of this “solarceramic stone” is under way to improve further the conversion efficiency for practical applications. Such an approach may also be applied to other layered semiconductive structures. The new report constitutes a part of an ongoing research portfolio in Professor Guo’s group for net-zero green fuels and chemicals.
Read the journal paper here.
Click here to learn more about Professor Zhengxiao GUO's group.