16 Jun 2025
Revolutionising Everyday Cleaners - A Greener Way to Produce Alkaline Hydrogen Peroxide

Hydrogen peroxide (H2O2) is a versatile chemical widely used for household cleaning, viral/bacterial sterilisation, textile bleaching, pharmaceuticals, wastewater treatment, chemical conversion, energy carriers, and semiconductor manufacturing. With a global market value of approximately 3.5 billion USD and a projected compound annual growth rate of 5%, its demand and significance continue to expand. However, the predominant production of H2O2, known as the anthraquinone method, is far from ideal. It is energy-intensive, environment taxing, and relies on hazardous materials. Additionally, this method requires chemical stabilisers to ensure the safe transportation of H2O2, further increasing its complexity and cost.
In a recent collaborative publication in Nature Communications, Professor Zhengxiao GUO of HKU Chemistry and Professor Junfeng LIU of Beijing University of Chemical Technology, have unveiled an eco-friendly and efficient alternative for H2O2 production from water. Their innovative method employs a highly efficient electrochemical approach based on the two-electron oxygen reduction pathway (2eORR). Unlike the conventional four-electron ORR (4eORR) that produces low-value oxygen gas, this approach selectively generates H2O2, enabling decentralised, on-demand production while reducing transportation risks, costs, and instability.
This breakthrough is achieved using a special catalyst, Ni-BDC (nickel-benzenedicarboxylic acid), which undergoes in-situ reconstruction during the reaction to form highly active sites. These sites have an optimised surface electronic structure, greatly enhancing the activity and selectivity for HO2⁻ (the deprotonated form of H2O2). Further studies reveal that residual ligand functional groups stabilise *OOH intermediates, which are crucial for improving the reaction's efficiency and selectivity.
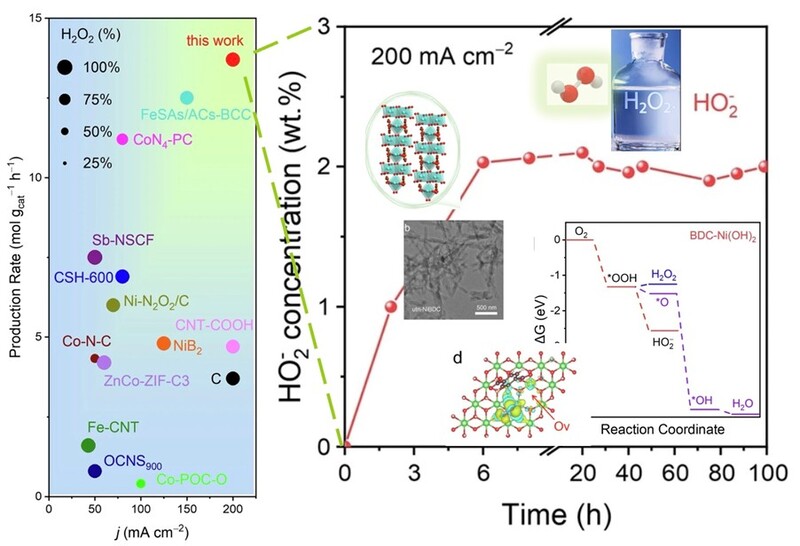
The catalyst achieves over 90% selectivity for HO2⁻ in alkaline conditions (0.1 M KOH) and operates stably across a wide current density range up to 200 mA cm⁻². It also delivers a high production rate of 13.7 mol gcat⁻¹h⁻¹ and accumulates 2.0 wt.% HO2⁻ over 100 hours of continuous operation. These results demonstrate its strong potential for large-scale industrial applications, as well as for eco-friendly household cleaning. For more details, please see: https://www.nature.com/articles/s41467-025-60467-0







