06 Jun 2023
Bridging a catalyst: HKU chemists developed a metal-free cocatalyst to promote facile oxygen reduction reaction by inducing the formation of key hydrogen bonds between surface species
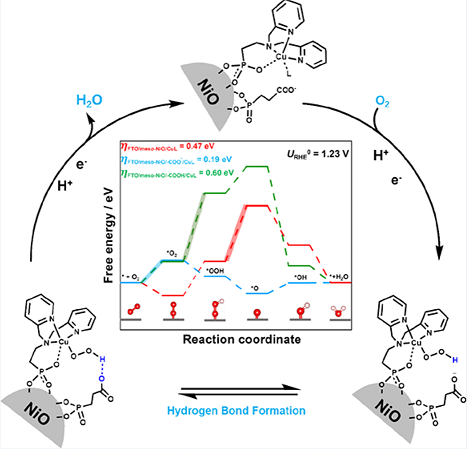
This scheme Illustrates efficient, selective oxygen reduction using earth-abundant electrocatalyst and new surface cocatalysts. Image adapted from ACS Catalysis, 2023, doi:10.1021/acscatal.3c00384.
Ceaseless efforts from extensive research are ongoing to establish a sustainable and reliable future energy source. Fuel cells are one of the promising technologies to promote switching from non-renewable sources to renewable ones. However, the sluggish oxygen reduction reaction (ORR) at the cathode hampers the overall electrochemical performance of a fuel cell by generating an unfavourable byproduct, which is H2O2.
A research group led by Dr Edmund CM TSE from the Department of Chemistry at the University of Hong Kong achieved an important breakthrough toward dismantling the technological barrier of fuel cells. Specifically, the Tse group has developed a promising electrochemical cocatalyst that overcomes the kinetic hindrances of ORR. The study was published on ACS Catalysis in an article entitled 'Immobilization of a Molecular Copper Complex and a Carboxylate Terminated Cocatalyst on a Metal Oxide Electrode for Enhanced Electrocatalytic Oxygen Reduction.'
A fuel cell cathode encounters challenges such as a high energy barrier for breaking the strong O=O bond and intertwined proton-coupled electron transfer (PCET) processes for ORR. Failure to solve these challenges could ultimately lead to the production of toxic H2O2. Introducing platinum as an electrocatalyst can partially address the kinetic issues by driving the four-electron four-proton ORR at a reasonable turnover rate but still with room for improvement. However, the rareness of precious metal has limited the practical usage of Pt, thus spurring the exploration of alternative pathways through developing non-precious metal-based catalysts. 'Copper is known as an earth-abundant transition metal that is used by nature in metalloenzymes to produce water exclusively without H2O2 generation.' Dr Tse recalled their initial design efforts. 'With these in mind, we have designed a bioinspired electrocatalyst featuring a Cu complex with dipicolylamine (DPA) ligand.'
Dr Shihuai WANG, a postdoctoral scholar in the Tse laboratory and the co-first author of this study, fabricated fluorine-doped tin oxide (FTO)-coated electrodes with mesoporous nickel oxide (meso-NiO) nanoparticles via a Ni sol-gel solution method. 'Our scanning electron microscope images show spherical pores with diameters varied between 20-60 nm.' Dr Wang elaborated on the unique features of their hybrid electrodes. 'These pores allow substrates easy access to the catalytic centre with a sophisticated surface coverage of functional moieties at the interface.' A copper(II) complex of functionalized DPA (CuL) and a carboxylate-terminated (COO–) phosphonopropionic acid (PPA) were then co-anchored onto the metal oxide surface of FTO/meso-NiO with the help of the phosphonate terminal group on the DPA ligand.
The electrochemical performance of the FTO/meso-NiO/PPA/CuL electrode in pH 7 phosphate buffer showed a catalytic current exceptionally higher than the control cases. Impressively, the FTO/meso-NiO/PPA/CuL electrode almost does not generate H2O2 byproduct. 'The dual-immobilizing scheme played a vital role in encouraging interactions between surface adducts to promote the favourable PCET route for O–O bond cleavage.' Dr Mo, another postdoctoral scholar in the Tse group, remarked on the sophisticated design of their heterogeneous electrochemical platforms. 'The on-surface synergistic effect facilitated hydrogen bond formation to boost the electrocatalytic activity and selectivity simultaneously.'
The Tse research team further explored the relationship between proton-coupled electron transfer steps and solution pH. 'At pH 7, the terminus of PPA converts from a neutral carboxylic acid group into an anionic carboxylate group.' Xutao Gao, a PhD student in the Tse group, performed density functional theory (DFT) calculations (Figure 1) to understand the working principle of this newly discovered cocatalyst. 'The negatively charged group then enticed the intermediates to form interfacial hydrogen bonds, thereby lowering the ORR energy barrier and releasing H2O as the sole product.' Distinct from the comparative control systems, the carboxylate-terminated FTO/meso-NiO/PPA/CuL catalyst showed a particularly low energy barrier of 0.19 eV due to the formation of hydrogen bonds.
By establishing a new paradigm in improving ORR activity and selectivity without direct modification of the non-precious metal catalyst structure, Dr Tse envisages that significant enhancement in fuel cell performance could be achieved via the inclusion of metal-free cocatalysts. This synergistic strategy is expected to be widely applicable to other green resourcification operations and clean energy catalytic processes.
Journal title: 'Immobilization of a Molecular Copper Complex and a Carboxylate Terminated Cocatalyst on a Metal Oxide Electrode for Enhanced Electrocatalytic Oxygen Reduction' (ACS Catalysis, 2023 )
The research paper can be accessed from here.
Click here to learn more about Dr Edmund CM TSE’s group.
Click here to learn more about HKU-CAS Joint Laboratory on New Materials.
More research stories:
HKU Chemists Developed Nitrile-Based Proton Carriers for Enhanced Oxygen Reduction