31 Oct 2020
HKU Chemists Developed Nitrile-Based Proton Carriers for Enhanced Oxygen Reduction
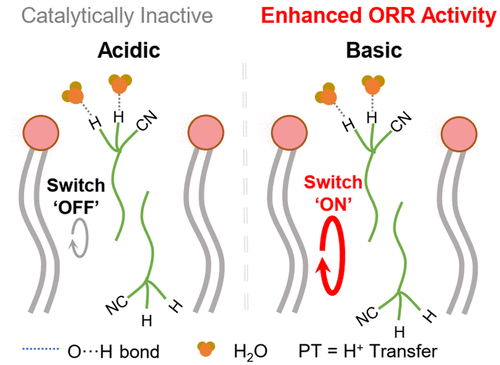
Nitrile-Facilitated Proton Transfer for Enhanced Oxygen Reduction by Hybrid Electrocatalysts. Image adapted from ACS Catalysis, 2020, doi:10.1021/acscatal.0c03506.
A research team led by Dr Edmund CM TSE from HKU Department of Chemistry has developed nitrile-based proton carriers that can facilitate transmembrane proton delivery to an HBM-supported copper (Cu) oxygen reduction reaction (ORR) catalyst under alkaline conditions. The stimuli-responsive proton regulators developed by the team can turn on the activity of the ORR catalyst on-demand, opening doors to investigate how proton transfer kinetics tune the selectivity of electrocatalysts for renewable energy conversion processes. The findings were published in ACS Catalysis.
'The development of efficient and sustainable energy conversion processes is a major challenge for scientists and researchers around the world.' Dr Tse discussed the outlook of clean technology powered by renewables. 'Fuel cells are one such technology that directly utilises chemically stored energy in fuels, such as hydrogen, to generate electricity.' However, the ORR that takes place at the cathode of the fuel cell is slow and requires a catalyst to facilitate the reaction for practical application. Platinum (Pt) is the best-performing catalyst to date, but its scarcity and high cost make it an impractical choice for widespread commercial use.
'We developed an experimental platform called hybrid bilayer membranes (HBMs) to study the electron and proton transfer steps.' Dr Tian ZENG, the first author of this work, explained the inner workings of this multilayer system. ‘The structure of an HBM is like a sandwich which consists of a lipid monolayer appended on top of a self-assembled monolayer (SAM).’ The SAM layer contains a non-precious metal catalyst featuring a Cu complex, which can rival the performance of their precious metal counterparts under alkaline conditions. Worthy of note is that Cu is much less expensive than Pt. The microenvironment surrounding the Cu catalytic site is further regulated by the HBM, where proton and oxygen are ferried from the solution to the embedded Cu centre located in the lipid interior by molecular carriers or passive diffusion.
In this study, nitrile-based functional proton transfer agents were found to turn ‘on’ and ‘off’ proton transfer on demand. Under acidic conditions, the proton switches are in the ‘off’ state, rendering the HBM-embedded Cu complex catalytically inactive. Under basic conditions, the proton carriers are switched ‘on’, prompting the Cu complex to exhibit enhanced ORR activity. Previously, the proton switch could only be turned ‘on’ upon acidification due to higher proton availability in low pH. For the first time, the proton gate can be toggled from the ‘off’ state to the ‘on’ state by adjusting the pH from 6 to 9 and vice versa during operation. This result signifies a major leap in building molecular protonics now that redox reactions can be turned ‘on’ in real-time in alkaline.
The work by the Tse group and collaborators from HKU and the University of Nevada, Reno, represents a new paradigm in artificial transmembrane proton transfer and closes the knowledge gap in designing smart devices. Dr Tse hopes that their breakthroughs will lead to more efficient energy conversion processes in the future, paving the way toward cleaner and greener power sources.
Journal Title: 'Nitrile-Facilitated Proton Transfer for Enhanced Oxygen Reduction by Hybrid Electrocatalysts' (ACS Catalysis, 2020)
The journal paper can be accessed here.
Click here to learn more about Dr Edmund CM TSE’s group.
Click here to learn more about HKU-CAS Joint Laboratory on New Materials.